Preimplantation Genetic Diagnosis
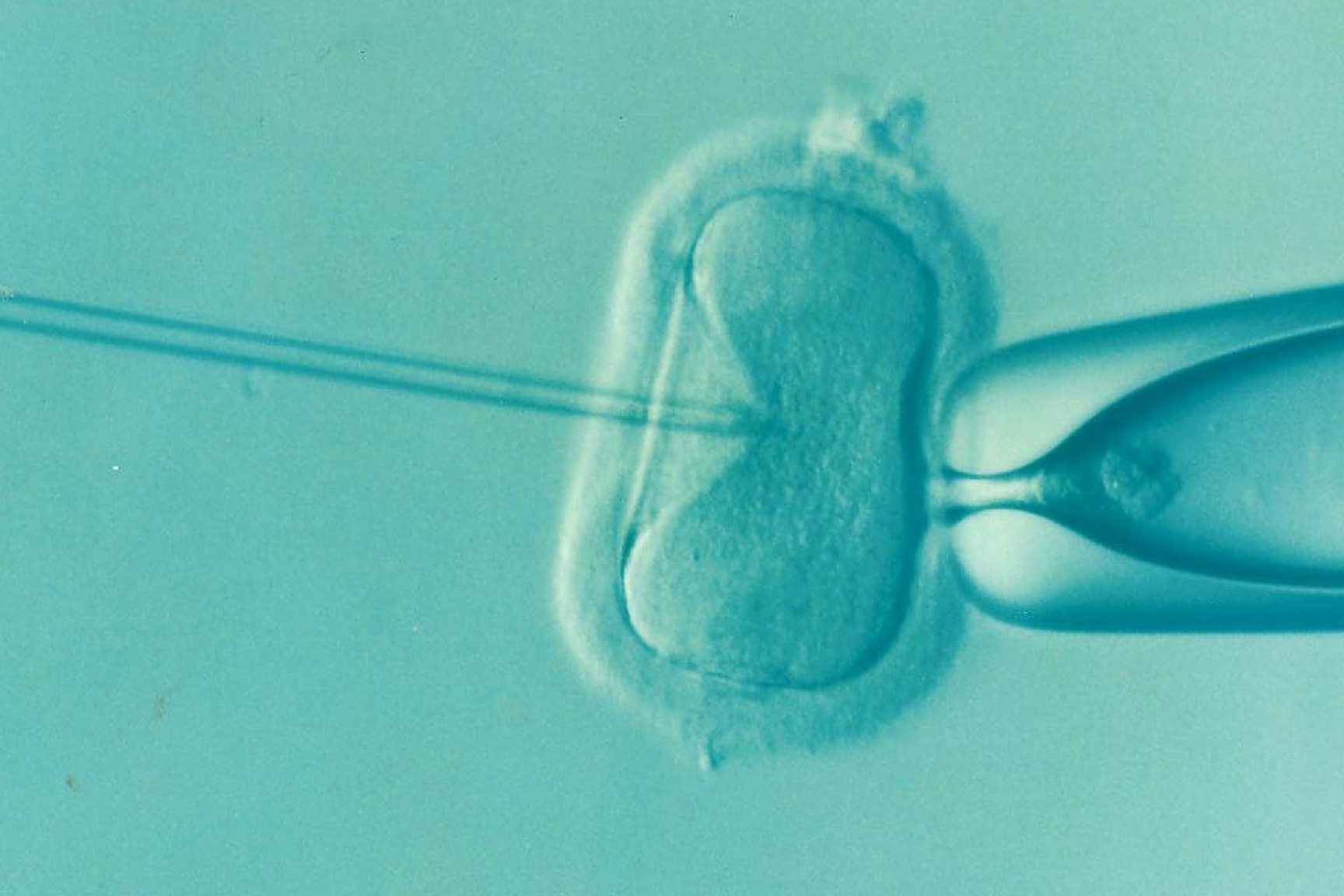
The term "preimplantation genetic diagnosis" (PGD) describes methods of testing embryos generated by in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI). The genome of one or two cells of a several-days-old embryo – usually during the so-called eight-cell stage (blastomere stage), i.e. about three days after fertilisation – is tested for certain mutations or chromosomal abnormalities that may result in a disorder, before the embryo is transferred to the uterus. PGD can also be used to identify other than disease-relevant characteristics, such as the embryo’s sex, the presence of a certain disability or the embryo’s suitability as an organ or tissue donor for a living sibling affected with a disease. In some countries, PGD is already being applied for these purposes. More indications for the application of PGD can be found in international medical literature: "high" maternal age, i.e. over 35 years, repeated unsuccessful IVF treatments, recurrent miscarriages and ICSI. However, a study published in 2007 in the New England Journal of Medicine claims that IVF with subsequent PGD does not lead to higher pregnancy rates.
In international medical literature, the indications for the application of PGD are: "high" maternal age, i.e., over 35 years, recurrent miscarriages, which may be due to chromosome disorders, and repeated ineffective treatment by in vitro fertilisation (IVF). In addition, PGD treatment is indicated in case of existing serious hereditary problems in the family due to monogenic diseases such as cystic fibrosis, myotonic dystrophy, spinal muscular atrophy, sickle cell disease, β-thalassemia, Huntington's chorea, epidermolysis bullosa or marker-X Syndrome. In the case of multifactorial diseases, many genetic risk factors in many embryos would have to be studied to find an embryo with a low risk for such a disease, which is currently not feasible.
In the case of the originally employed methods the one or two cells which are removed from the embryo for testing are totipotent at this stage. It can thus be assumed that, given certain conditions, they could each develop into an individual embryo. As the destruction of totipotent cells is considered as ethically and legally problematic (see below), the removal of cells (biopsy) is increasingly performed five to six days after fertilisation, i.e. at the so-called blastocyst stage. According to the current state of research, these cells are no longer totipotent, but merely pluripotent. Furthermore, it is assumed that this form of biopsy is less invasive because the trophectoderm of the blastocyst is examined, the cells of which subsequently develop into the placenta and amniotic membrane. Another advantage is that a smaller proportion of the total cell mass is taken, but in total more cells can be examined, which increases the certainty of the diagnosis.
The PGD-related methods of preconception or prefertilisation diagnosis are less controversial than the genetic testing of embryos. They involve the genetic analysis of the polar bodies of the woman’s oocytes prior to fertilisation. This method thus only provides information on the maternal genotype. Moreover, because the costs are higher and two biopsies have to be done instead of one compared to blastomere or blastocyst biopsy, polar body diagnostics are increasingly replaced by other types of PGD. Preconception diagnostic procedures could be complemented by a preconceptional genetic analysis of the male spermatocytes. However, the main difficulty of this method lies in the fact that the spermatocytes are generally being destroyed in the procedure and are thus no longer available for later fertilisation of an oocyte. Currently, procedures aiming at artificially duplicating the spermatocytes prior to their genetic analysis are being clinically tested: thus, the genetic material of the first spermatocyte could be used for testing and the second, identical spermatocyte for fertilisation. There are also physical methods that allow sperm to be examined for the sex chromosome. This makes it possible to exclude certain sexchromosome-linked diseases.
Physical methods allow the identification of sperms carrying Y-chromosomes, as they vary in mass to those carrying X-chromosomes due to different DNA contents. This could be used in the context of fertilisation involving sex selection to avoid X-linked genetic diseases.
If PGD and the subsequent transfer of the embryos to the uterus result in a pregnancy, the diagnostic findings of PGD are usually checked by the (non-invasive and/or invasive) methods of prenatal diagnosis (PND).
The risks of PGD for the woman can be compared to those of extracorporeal fertilisation by IVF/ICSI without PGD. On the one hand, the removal of the oocytes and the transfer of the embryos following PGD may bring about infections. On the other hand, there is an increased risk of ovarian hyperstimulation syndrome. This is due to the fact that, as a general rule, more oocytes have to be generated for PGD than for IVF/ICSI without PGD, given that many embryos are not transferred because of unsuccessful biopsies, diagnostic findings or irrelevant results of the testing. Therefore, hormonal stimulation is usually set at a higher level when PGD is used than in the context of in vitro fertilisation without subsequent PGD. In addition, there are risks for mother and child related to multiple pregnancies, which increasingly occur in the context of extracorporeal fertilisation. Moreover, the woman or the couple may have to face high psychological strain as a result of hormone treatments and also of hopes and fears related to the success of the method.
The embryo faces the risk of not being transferred and of being destroyed in the case of abnormalities discovered, irrelevant testing results or false diagnosis. Further risks include the embryo’s destruction during the removal of cells for testing and the increase in multiple pregnancies. Some studies prove that IVF embryos are more likely to develop malformations.
In addition to PGD, procedures are being studied which, by means of the significantly improved possibility of human gene editing, shall alter the DNA of embryonic cells showing hereditary disposition for monogenic diseases such as myotonic dystrophy or Huntington’s disease. While until now such embryos are discarded within the course of PGD they could be employed after a successful alteration of their genetic sequences which includes, among others, the cutting out of "defective" genes. These procedures are so far experimental. Moreover, the related alteration of the human genome is subject to legal restrictions and ethical concerns.
Legal regulation in Germany
In Germany, the provisions for PGD are regulated in the framework of the embryo protection act (Embryonenschutzgesetz (ESchG)). With the law of regulation of the preimplantation genetic diagnosis (Präimplantationsdiagnostikgesetz (PräimpG)), which got approved by the German parliament on November 21st 2011, and the change of the embryo protection act related to it, and despite its fundamental prohibition, the genetic examination of the pluripotent cells of the embryo in vitro, before its intrauterine transfer, within exceptions and tight limits, is declared not illegal. Hence there is an explicitly legal regulation of PGD for the first time. Applying PGD on the basis of the new law is regulated by the ordinance on the legitimate implementation of preimplantation genetic diagnosis (Präimplantationsdiagnostikverordnung (PIDV)), which came to force on February 1st 2014.
PGD involves the testing of embryos and unfertilised oocytes and, depending on the results of the analysis, their exclusion from the reproductive process before the achievement of a pregnancy is being attempted. Several main emphases can be distinguished in the current debate on the ethical assessment of PGD.
The ethical debate on the methods of PGD centres on the question whether the embryo’s possible rights to protection are being violated by these methods, and if so to what extent. Underlying this discussion is the question of the moment from which rights and protection are assigned to the embryo. This question is not only relevant with regard to the manipulation of the embryo during the biopsy and the inevitable destruction of embryonic cells during the diagnosis, but also and especially when it comes to the possible decision not to transfer the embryo to the uterus due to embryopathy or other undesired characteristics.
Decisive for an assessment of the moral status of the human embryo is the respective underlying ethical concept of protection. Two basic positions can be distinguished. A first position fully transfers the entitlement to protection associated with a human being after birth to the human embryo from its very beginning, i.e. from the fusion of nuclei, due to its personhood. A second position grants the embryo a graduated entitlement to protection depending on its achievement of a certain developmental stage. Possible relevant developmental moments are the nidation in the uterus, i.e. the moment from which an embryo is actually capable of developing, or the formation of the primitive streak which marks the completion of individuation, as from that moment the possibility of a multiple pregnancy can be ruled out. The arguments given in scientific discourse regarding ontological prerequisites for full or graduated entitlement to protection can be assigned to four main categories. In Germany, they are often called SKIP-arguments (from the initial letters of the relevant headwords in German). They are supported or challenged individually, but also in combination, as they complete each other and are interdependent.
- Species argument
According to the species argument, embryos, since they biologically belong to the species Homo sapiens, possess dignity and are thus worthy of protection like all other members of this species. In the sense of equal treatment, they have the same right to life as born human beings. - Continuity argument
The continuity argument is closely linked to the species argument when it comes to the question of the biological beginning of individual human life. It is based on the assumption that embryos continuously develop and that in the process of their development into a born human being there are no breaks of moral relevance; consequently they have to be afforded the same rights as born human beings. - Identity argument
The thesis underlying the identity argument states that the embryo’s dignity can be derived from the morally relevant, existing identity between a born human being and the embryo from which he or she has developed after the fusion of spermatocyte and oocyte and from the fact that the born possesses human dignity. - Potentiality argument
The potentiality argument implies that the fertilised oocyte already possesses the full potentiality to become a born human being. In the natural process of anthropogenesis, the embryo thus has, since its beginning, the potentiality to develop into a personal being and a moral subject. Due to this potential, existing from the beginning, the embryo is worthy of unrestricted protection.
Furthermore, as regards the methods of PGD, the ethical discussion with respect to the application of extracorporeal fertilisation techniques as a prerequisite for PGD is cast in a new light. Critics assert that their admissibility used to be bound to other prerequisites and objectives. In the context of their application for PGD however, they are used for couples that are also able to give birth to children without the methods of assisted reproduction. This means that the scope of application of these techniques is changing. Whereas in the original field of application the establishment of a pregnancy and thus ultimately the birth of a child is the objective, in the context of PGD they are used to select embryos with specific diagnostic findings. Thus, selection becomes the prime objective, while pregnancy and the birth of a child who shall not bear a certain characteristic come only second.
With the PGD several objectives of the application are pursued. The ethical debate centres on the question whether, and if so to what extent, these objectives justify possible violations of the embryo’s right to protection. A current objective of the application is the increase in the chances of having offspring without the risk of passing on genetic diseases. In this context for example a case from the UK is discussed in which a couple received PGD in order to prevent passing on a genetic form of breast cancer. In an ethical perspective in terms of the objective of the application discussions are going on especially about the difficulty of choosing the diseases or characteristics that ought to be diagnosed respectively the characteristics or criteria of selection (fatal diseases, late-onset diseases, untreatable diseases etc.).
Another objective of the application of PGD is the production of so-called saviour siblings. They are children with respect to their suitability as a tissue donor to help out an older sibling for their treatment. For this purpose the embryo with the highest genetic congruence with the ill child gets implanted after an in vitro fertilisation via PGD. After giving birth the stem cells of the cord blood or the bone marrow of the new born shall help the ill sibling. Supporters of this field of application assert that, in such a case, PGD allows to help a child affected with a life-threatening disease, for whom no other compatible donor can be found. Critics object to this that an embryo is not created primarily for its own sake, but with the aim of helping another human being, this leading to complete instrumentalisation which cannot be ethically justified. Moreover, and especially with regard to further treatments, this gives rise to the question of whether a child who is brought into the world for this purpose would have the opportunity to make a decision of his or her own free will in favour of or against helping the sibling and possibly suffering the pain involved. What is more, a considerable number of healthy embryos is generated and rejected again.
In the context of this discussion, it is of substantial importance whether there are no other possibilities of treatment that might be successfully applied, whether a fatal disease is to be treated and whether the child born after PGD will possibly have to repeatedly undergo painful operations. A third objective of the application concerns about the selection of embryos for non-disease-related characteristics which are rejected as ethically problematic, e.g. the embryo’s sex. However, supporters of this possible application of PGD argue that also social criteria should be admissible. Especially with regard to non-disease-relevant characteristics, PGD has raised fears of a slippery slope to eugenic selection and ethically disputed research on embryos.
The consequences of authorising PGD, i.e. social consequences as well as consequences for disabled and non-disabled individuals, are also often discussed: with regard to social consequences, there are fears of an increasing discrimination against sick and disabled persons. If the methods of PGD were authorised, couples who wish to have a child could be subject to social pressure to prevent disabled life, suggesting that parents of potentially disabled children should use the methods of PGD for the common good and in order to avoid higher costs related to care and assistance for a disabled or sick child to society. The mechanisms that could result from this would not increase reproductive freedom which is often cited in the context of PGD, but, on the contrary, make it more difficult. Some say, this has already become visible in the example of PND: the availability of the methods alone leads to social pressure to use them as well. Critics also assert that an authorisation of PGD would result in "unworthy of life"-judgements being approved. This would have a serious impact, especially on the life situation of disabled and sick persons. In the case of PGD being authorised, they would be confronted with an officially tolerated "unworthy of life"-judgement which would radically question their own existence.
Other areas of discussion concern questions that deal with the autonomy of the couple, the role and the concept of woman, the doctor-patient relationship (in this respect, the couple, the woman and/or the embryos are considered as patients) and adequate advice and assistance for the couple, for the woman in particular, but also for the health personnel.